We study B cell immunity during anti-viral responses, with focus on upper respiratory tract
We are particularly interested in defining the key forces driving immunodominance following influenza virus infection and vaccination.
We use a combination of flow cytometry, microscopy, immunological assays, antibody and single-cell sequencing methods.
Our ultimate goal is to apply this knowledge in order to improve current and develop new vaccines.
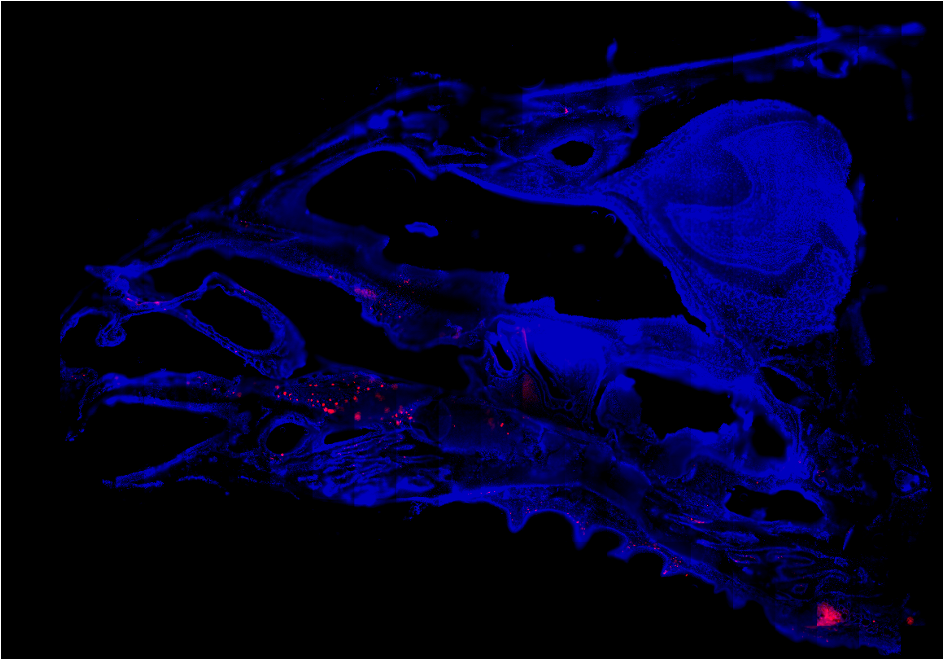
Mucosal Immunity in the Respiratory Tract
Influenza A Virus primarily replicates in the upper and lower respiratory tract. In recent years, tissue resident
memory B cells (BRM) were shown to be established in the lungs after infection. These cells rapidly reactivate after re-infection
to differentiate to antibody secreting cells.
In the lab we address several gaps of knowledge in airways immunology: ongoing projects aim at defining the exact factors required
for lung BRM formation and maintenance.
A strong focus of the laboratory is also on adaptive immunity in the upper respiratory tract, including nasal tissue and NALT. Immunology
in the nasal tissue is almost uncharted territory and our current projects aim at investigating B and CD4 T cell dynamics in
this niche. By applying a restricted infection method, we have discovered robust adaptive immune responses ongoing in these tissues.
Key Publications:
Romain´s study discovered the
presence of ectopic germinal centers in the nasal tissue of mice and humans.
Nimitha´s work defined
origin and transcriptional program of lung BRM
Nimitha´s preprint identified a population of
influenza-specific CD4 TRM in the nasal tissue of mice and humans.
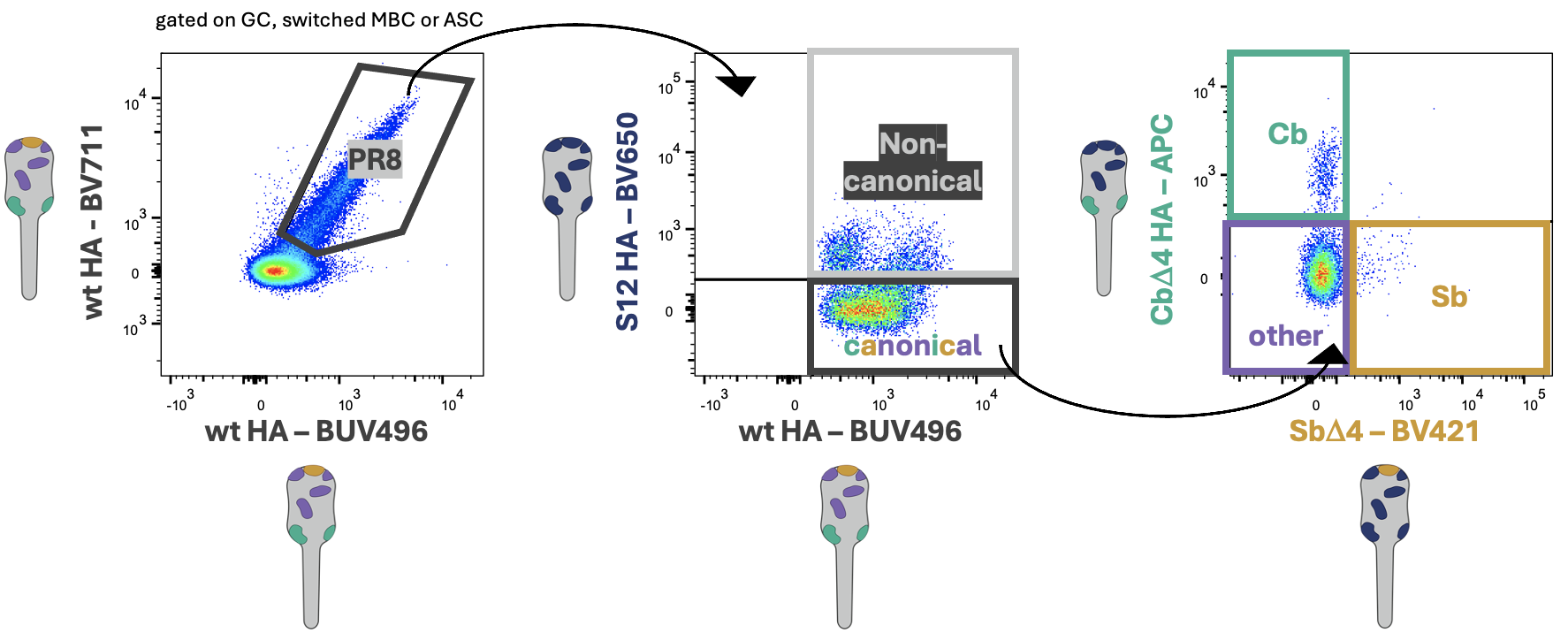
Effect of Pre-existing Immunity on Recall of Memory B Cells
Pre-existing immunity influence subsequent immune responses. Humans are continuously exposed to influenza virus
infections and vaccinations with slightly mutated viruses. In recent years, many studies have elucidated the
mechanisms underpinning memory B cell reactivation and germinal center re-entry.
In this project we are taking advantage of unique reagents generated in our lab that can break down antibody and
B cell specificity to the antigenic site level. Using these, combined with advanced fate-mapping models
and polyclonal memory B cells and antibody transfers,
we have been able to unravel how presence of very well defined immune-components can stir
memory B cell re-entry in germinal centers vs antibody secreting cell differentiation.
Key Publication:
Danica´s paper described the role of pre-existing CD4 help in shaping B cell responses
Laura´s preprint dissected B cell immunodominance during secondary immune responses
Novel Vaccines and Therapeuthics
There is urgent need of novel vaccines and therapeutics that can protect against seasonal and pandemic threats.
For example, the current H5N1 spread within wild birds and cattle in the United States is causing heightened concerns
for the potential of human spillover.
In the lab we collaborate with world leading laboratories to effectively design and test novel vaccines and therapeutics.
Current projects include the computational design of immunogens that mimic epitopes of broadly-neutralizing anti-influenza
antibodies. In addition, we have isolated a nanobody specific for the avian influenza strain H7N9 and discovered that this
nanobody was also able to cross-recognize and cross-protect against several viral variants similar to the ones circulating in humans.
Key Publications:
Zhao-Shan´s study identified a broadly neutralizing nanobody
Laura´s collaborative preprint defined the ability of
a computationally designed stem-mimetic immunogen to induce influenza-specific antibodies with broad binding capacity.
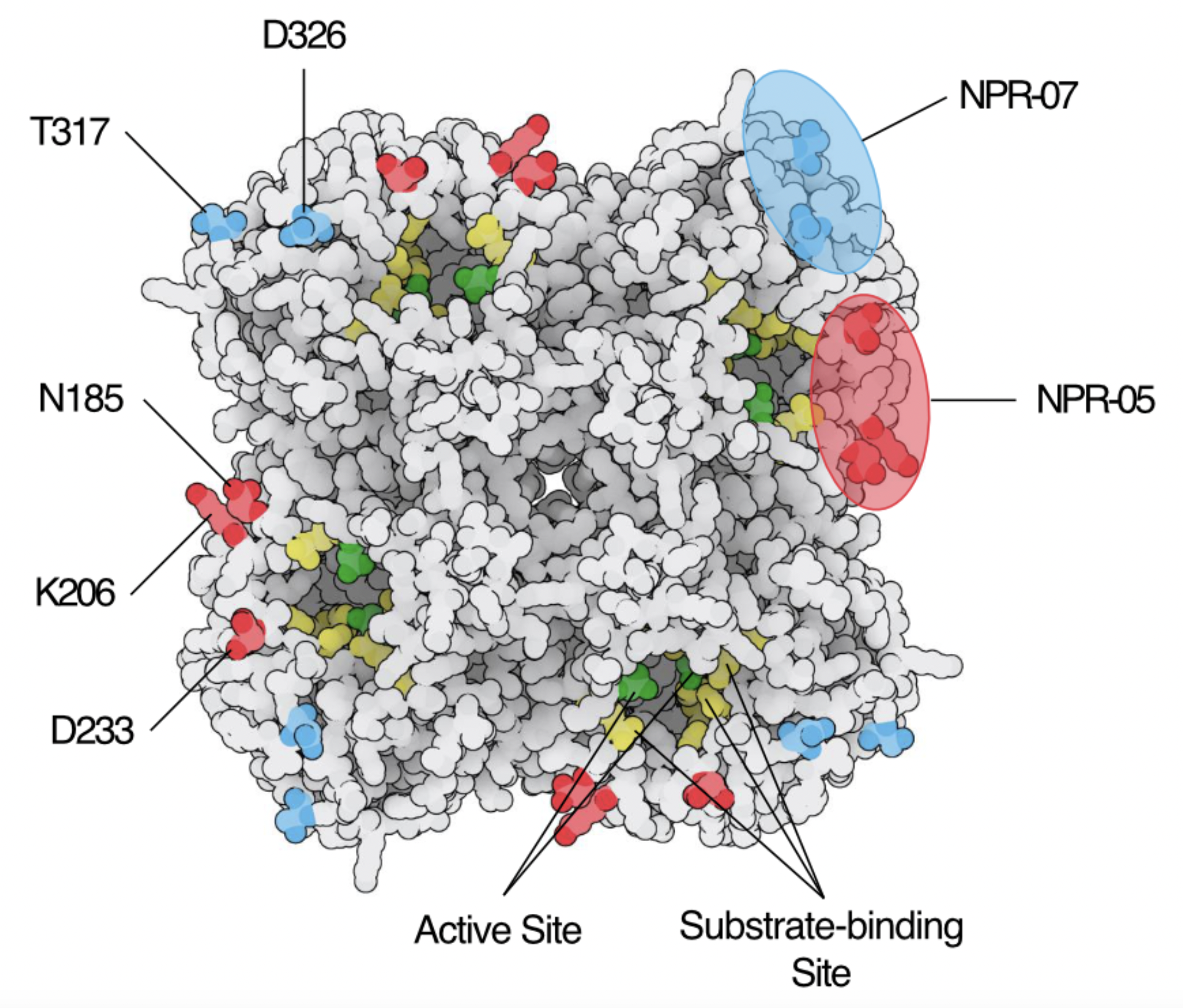
Neuraminidase Specific Antibodies
Neuraminidase (NA) is the second major surface glycoprotein of influenza A virus. Until recent, NA has been neglected and, indeed,
its amount is still not measured in seasonal vaccine preparations. However, antibodies to NA have proven to be equally if not even
more potent as compared to hemagglutin-specific antibodies. Furthermore, with NA being an enzyme essential for virion release,
escape is not as common.
In our lab we are trying to define properties and protective potential of NA-specific antibodies. In our current work, we have
isolated and characterized a number of influenza PR8-specific monoclonal antibodies. To this end we have adopted and modified
the MUNANA assay, an enzymatic assay widely used to study NA-activity.
In addition, we are exploring how antibody isotypes can affect their anti-NA activity in relevant in vitro ALI models and
in vivo
Key Publications:
Ilya and Danica´s paper identifies PR8 NA-specific mAbs and characterize them using kinetic MUNANA assay
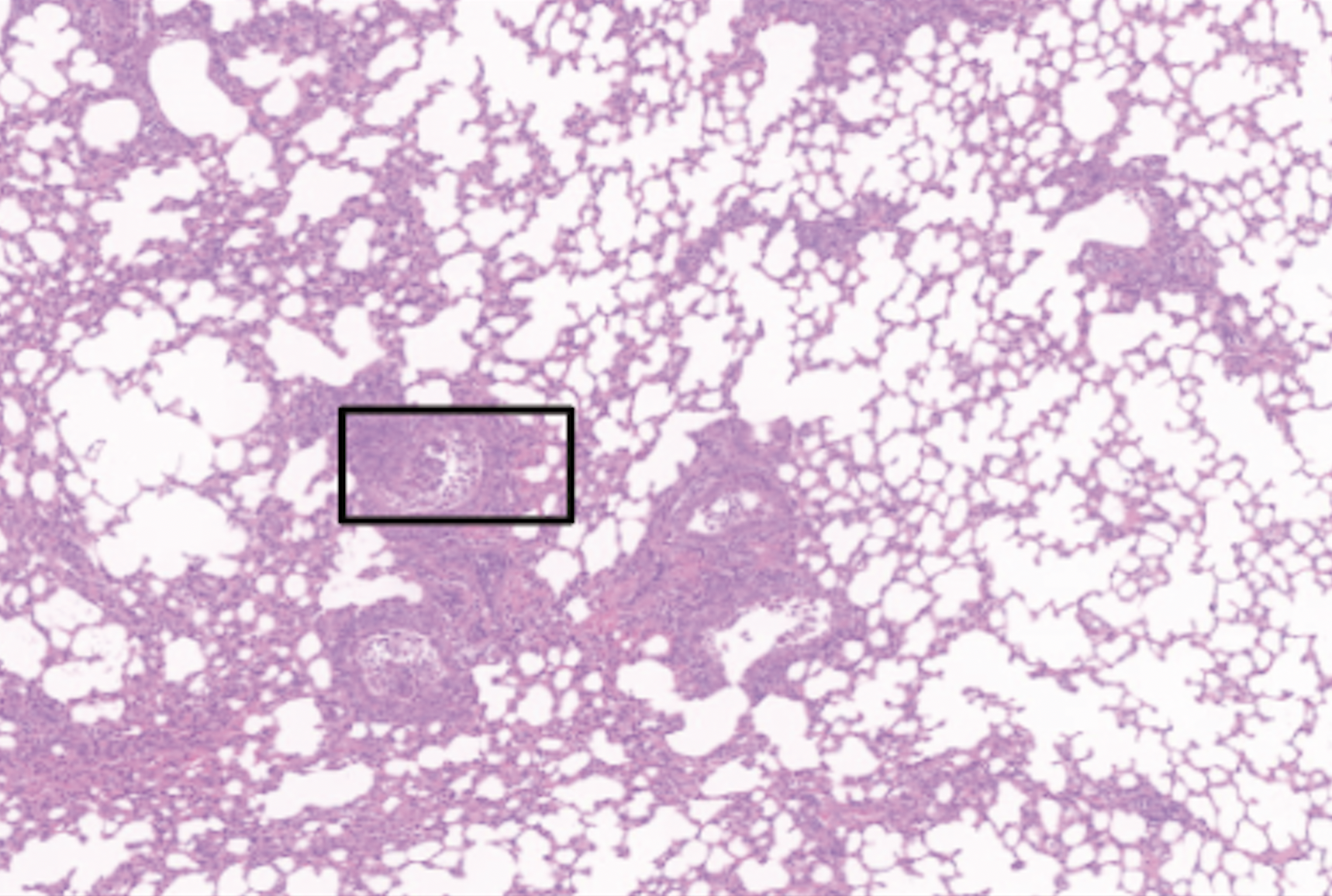
Other Ongoing Projects
We are exploring many other aspects related to B cell immunology, antibody biology but also virology and viral immunology.
Some examples below:
In one of our study, we have investigated how influenza infection can relocalize Argonaute-2 (AGO2) protein to the nucleus.
AGO2 is a major player involved in RNAi. In our work, we discovered how nuclear AGO2, after viral infection, silences type-I-interferon
related genes thus favoring viral proliferation.
We are also studying how transcriptional regulations affects B cell differentiation in different models,
including vaccination, infection and cancer.
Finally, we are looking into developing synthetic virology approaches to facilitate reverse genetics.
Key Publications:
Hsiang-Chi´s article on nuclear AGO2 after influenza infection
Collaborative work with Astra Zeneca, where we describe a
transcriptional signature that is intimately linked to B cell afinity.
